Technical Contents
Engineering Guide: Medical Plastic Injection Molding
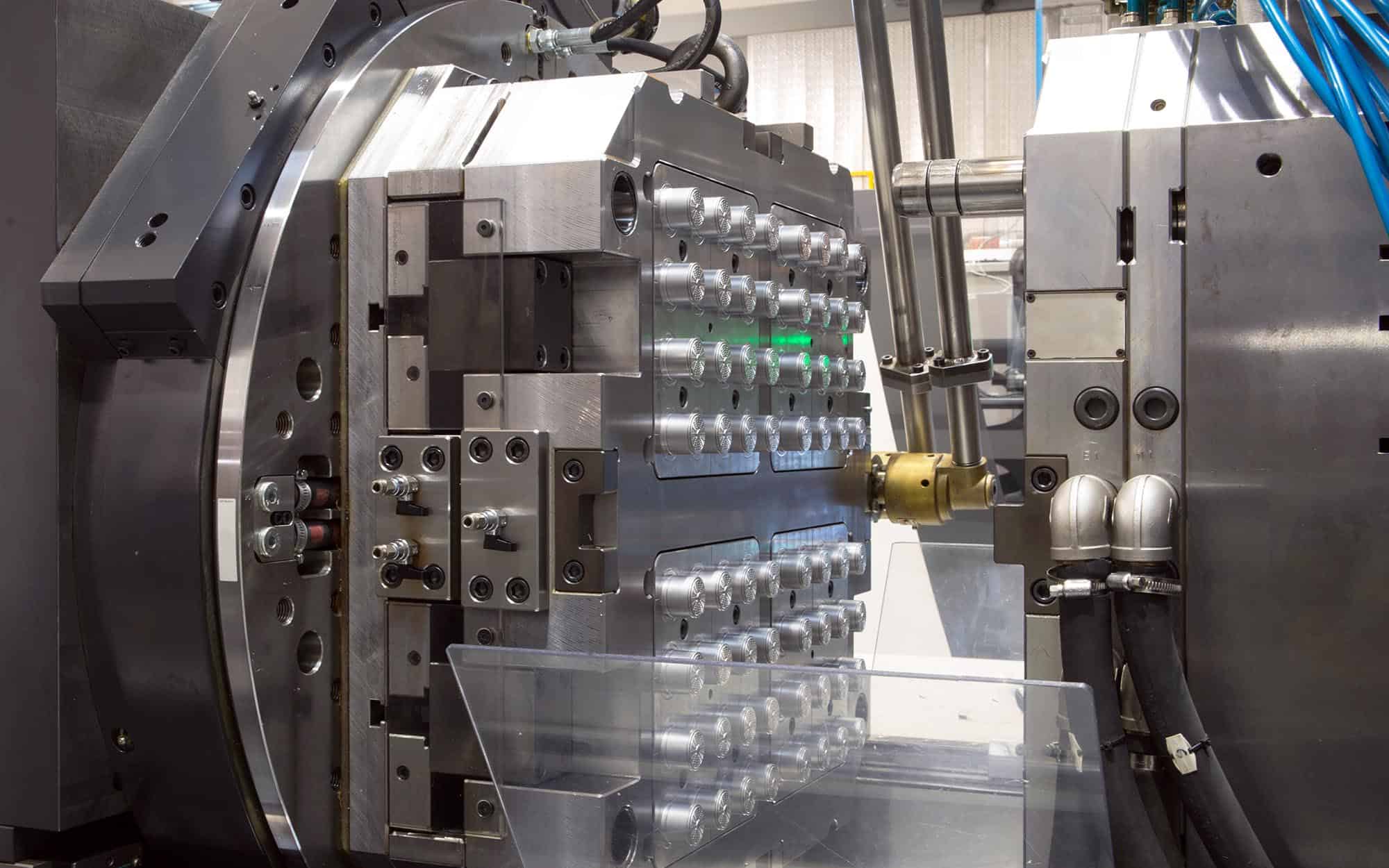
Engineering Insight: Precision in Medical Plastic Injection Molding
In the field of medical device manufacturing, precision is not merely a performance metric—it is a regulatory and functional imperative. Plastic injection molding for medical applications demands micron-level accuracy, repeatable process control, and compliance with stringent biocompatibility standards. At Wuxi Lead Precision Machinery, we apply our deep expertise in high-tolerance metal manufacturing to support the production of advanced injection molding systems used in medical component fabrication. Our engineering heritage, forged through mission-critical projects for Olympic athletic equipment and military defense systems, directly informs our approach to precision in medical molding technology.
Medical plastic injection molding involves the production of components such as syringe bodies, inhaler housings, surgical instrument housings, and implantable device enclosures. These parts must meet ISO 13485 quality standards, often require Class 8 (100,000) cleanroom production environments, and must consistently achieve tight tolerances—typically within ±0.025 mm. Variability at the micro level can compromise device function, patient safety, and regulatory approval. This level of consistency is only achievable through precision-engineered molds, advanced process monitoring, and thermally stable machine platforms.
Our experience in custom metal manufacturing for high-stakes industries has equipped us with the technical rigor necessary for medical molding applications. Components developed for Olympic athletes required flawless repeatability under extreme conditions, while military contracts demanded zero-failure performance in harsh environments. These same principles—robust design, material integrity, and dimensional stability—are central to the molds and support systems we engineer for medical injection molding.
Wuxi Lead Precision Machinery specializes in the fabrication of mold bases, cavity inserts, and precision guide systems using high-grade tool steels such as H13, S136, and 420 stainless, all processed through CNC milling, wire EDM, and surface grinding to ensure geometric accuracy. Our in-house metrology lab employs coordinate measuring machines (CMM) and laser interferometry to validate tolerances, ensuring every component meets the exacting demands of medical production.
We collaborate closely with medical device manufacturers and mold builders to deliver metal components that form the backbone of reliable injection molding systems. By integrating thermal management features, wear-resistant coatings, and alignment systems with sub-micron repeatability, we help reduce cycle times, minimize flash, and extend mold life—critical factors in cost-effective, high-quality medical manufacturing.
| Specification | Detail |
|---|---|
| Typical Tolerance Range | ±0.005 mm to ±0.025 mm |
| Material Options | H13, S136, 420 Stainless Steel, SKD61 |
| Surface Finish | Ra 0.05 µm to Ra 0.4 µm (polished to mirror grade) |
| Process Capabilities | CNC Milling, Wire EDM, Surface Grinding, CMM Inspection |
| Cleanroom Compatibility | Yes (supports Class 8 environments) |
| Certification Standards | ISO 9001, ISO 13485 (via partner facilities) |
Precision in medical plastic injection molding begins with the metal components that form the mold. At Wuxi Lead Precision Machinery, we ensure that every machined part contributes to a system capable of delivering life-critical components with absolute reliability.
Precision Specs & Tolerances
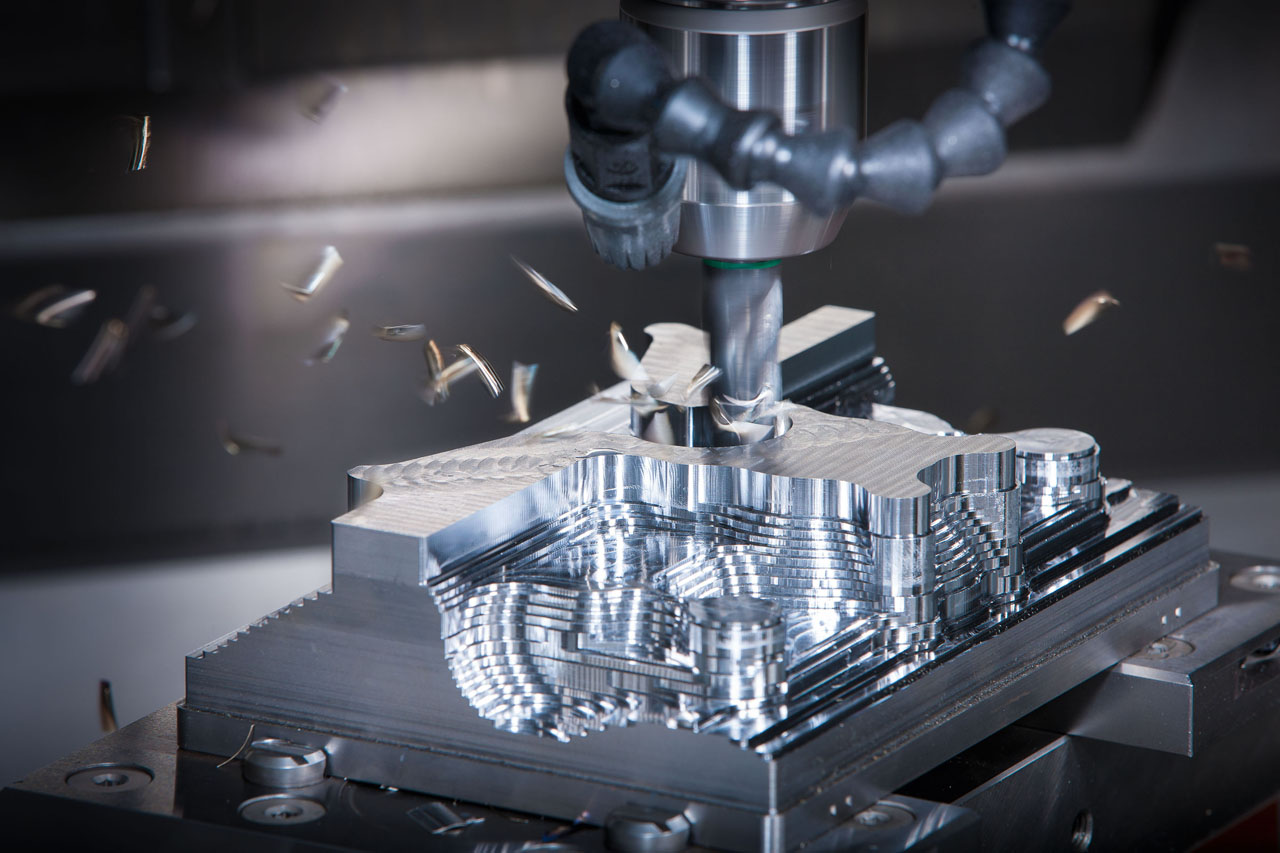
Technical Capabilities for Medical Plastic Injection Molding Support
Wuxi Lead Precision Machinery delivers critical metal components enabling precision medical plastic injection molding. Our core expertise lies in manufacturing ultra-precise mold bases, core/cavity inserts, hot runner systems, and ejection components from medical-grade tool steels and alloys. This foundational metalwork directly determines the dimensional accuracy, surface finish, and longevity of the final plastic medical device. We operate under strict ISO 13485-aligned protocols, ensuring every machined part meets the stringent biocompatibility and performance demands of Class I, II, and III medical devices.
Our advanced 5-axis CNC machining centers form the backbone of this capability. These systems enable single-setup machining of complex, multi-faceted geometries essential for intricate mold cavities and undercuts found in devices like surgical instrument housings, fluid pathway components, and microfluidic chips. Simultaneous 5-axis movement ensures superior surface integrity and eliminates cumulative error from multiple fixturing stages. Material compatibility spans P20, H13, S136 stainless, and specialized maraging steels, processed with optimized toolpaths to minimize thermal distortion and maintain critical hardness post-heat treatment.
Quality control is non-negotiable in medical manufacturing. Every component undergoes comprehensive CMM inspection using calibrated Zeiss and Hexagon systems. Our inspection regime verifies all critical dimensions against CAD models, including complex contours, radii, and positional tolerances. Surface roughness is quantitatively measured to ensure optimal release properties and prevent cosmetic defects in the molded plastic part. Full inspection reports with traceable data points are provided, supporting client regulatory submissions and internal quality audits. This rigorous metrology validates that mold components will consistently produce plastic parts within the tightest medical specifications over extended production runs.
The following table details our standard and medical-grade tolerance capabilities for critical mold features:
| Tolerance Type | Standard Capability | Medical-Grade Capability | Measurement Method |
|---|---|---|---|
| Linear Dimensions | ±0.010 mm | ±0.005 mm | CMM |
| Flatness | 0.010 mm | 0.003 mm | CMM / Optical Flat |
| Parallelism | 0.015 mm | 0.005 mm | CMM |
| Perpendicularity | 0.015 mm | 0.005 mm | CMM |
| Positional (Holes) | ±0.010 mm | ±0.003 mm | CMM |
| Surface Roughness (Ra) | 0.4 µm | 0.2 µm | Profilometer |
This precision engineering capability ensures mold longevity exceeding 1,000,000 cycles while maintaining part consistency. We partner with medical molders to translate complex device requirements into robust, high-performance metal tooling solutions, directly impacting the success of your injection molding process and final product quality. Our technical team provides full DFM support to optimize manufacturability without compromising medical performance criteria.
Material & Finish Options

Material Selection for Medical Plastic Injection Molds: Aluminum, Steel, and Titanium
Selecting the appropriate base material for medical plastic injection molds is critical to ensuring part quality, production efficiency, and regulatory compliance. At Wuxi Lead Precision Machinery, we specialize in custom metal manufacturing for high-precision applications, particularly in the medical device sector where cleanliness, repeatability, and dimensional stability are paramount. The choice between aluminum, steel, and titanium depends on production volume, part complexity, sterilization requirements, and mold longevity.
Aluminum alloys, particularly 7075 and 6061-T6, are widely used for prototype and low-to-medium volume production molds. Their excellent thermal conductivity allows for faster cooling cycles, reducing cycle times and increasing throughput during early-stage manufacturing. Aluminum is also easier to machine, enabling complex geometries to be produced with tight tolerances. However, its lower hardness compared to steel makes it less suitable for long production runs or abrasive medical-grade resins.
Tool steels such as P20, H13, and 420 stainless steel are the standard for high-volume medical molding applications. These materials offer superior wear resistance, hardness, and durability. 420 stainless steel is especially valued in medical molding due to its corrosion resistance, essential for molds exposed to aggressive cleaning agents and repeated sterilization cycles. With proper heat treatment, steel molds can withstand millions of cycles while maintaining dimensional accuracy and surface finish integrity.
Titanium alloys, though less common, are gaining traction in specialized applications where extreme corrosion resistance, high strength-to-density ratio, and biocompatibility are required. While titanium is challenging to machine and more costly, its performance in highly corrosive environments or for implantable device tooling justifies its use in niche medical applications.
Surface finishing, particularly anodizing for aluminum molds, plays a vital role in mold performance. Hard anodizing increases surface hardness, improves wear resistance, and enhances corrosion protection. For medical molds, anodized layers must be fully sealed to prevent particle shedding and ensure cleanability. In steel molds, electroless nickel plating or polished chrome finishes are often preferred, but anodizing remains exclusive to aluminum substrates.
Below is a comparative overview of key material properties relevant to medical injection molding:
| Material | Hardness (HRC) | Thermal Conductivity (W/m·K) | Corrosion Resistance | Typical Use Case |
|---|---|---|---|---|
| Aluminum 7075 | 40–45 | 130 | Low to Moderate | Prototypes, low-volume production |
| P20 Tool Steel | 28–32 | 30 | Moderate | Medium to high-volume molds |
| H13 Steel | 48–52 | 32 | Moderate | High-stress, high-temperature molding |
| 420 Stainless | 50–54 | 25 | High | Sterilizable, corrosive environments |
| Titanium Grade 5 | 35–40 (equiv.) | 7 | Very High | Specialized, corrosive applications |
At Wuxi Lead, we guide clients through material and finish selection based on application demands, ensuring optimal performance, regulatory alignment, and cost-efficiency across the product lifecycle.
Manufacturing Process & QC

Medical Plastic Injection Molding: Precision Production Process
Medical plastic injection molding demands uncompromising precision due to stringent regulatory requirements and zero-tolerance for defects in patient-critical components. At Wuxi Lead Precision Machinery, our end-to-end process integrates engineering rigor with advanced manufacturing controls to ensure every part meets ISO 13485 and biocompatibility standards. The journey begins with Design for Medical Manufacturability (DFM), where our engineering team collaborates with clients to optimize part geometry, material selection (e.g., USP Class VI resins), and mold flow analysis. We prioritize features like draft angles, wall uniformity, and gate placement to eliminate weld lines or sink marks that could compromise sterility or structural integrity. Finite element analysis (FEA) validates stress points under clinical use conditions, ensuring compliance with ASTM F2095 leak-test criteria before tooling commences.
Prototyping utilizes hardened steel molds—not soft aluminum—to mirror production conditions. This phase validates dimensional accuracy within ±0.025 mm tolerances and surface finishes down to Ra 0.1 µm, critical for implants or fluid-handling devices. We conduct iterative trials with certified medical-grade polymers, monitoring cavity pressure curves and thermal stability to detect micro-variation risks. Each prototype undergoes rigorous first-article inspection (FAI) per AS9102 standards, including CMM scans, material lot traceability, and functional testing against FDA 21 CFR Part 820 requirements. This eliminates the false confidence of “prototype-only” tooling, ensuring seamless scale-up.
Mass Production executes zero-defect manufacturing through closed-loop process control. Our all-electric injection molding platforms (e.g., Toshiba IS Series) maintain pressure stability within ±0.1% and temperature variance under ±0.5°C. Real-time sensors track 20+ parameters per cycle, with AI-driven SPC (Statistical Process Control) halting production if deviations exceed pre-defined control limits. Automated vision systems inspect 100% of parts for cosmetic flaws, flash, or dimensional drift, while in-process material drying ensures moisture levels stay below 0.02% to prevent hydrolysis. Every batch includes full material traceability—from resin pellet lot to finished part—with documentation for MDR/IVDR compliance.
Key process specifications ensure medical-grade consistency:
| Parameter | Prototyping Phase | Mass Production Phase |
|---|---|---|
| Tolerance Control | ±0.025 mm | ±0.015 mm |
| Cavity Pressure | ±0.5% stability | ±0.1% stability |
| Surface Finish | Ra 0.1–0.8 µm | Ra 0.05–0.4 µm |
| Cavity Count | 1–2 cavities | 8–32 cavities |
| Inspection Scope | 100% FAI + functional | 100% automated vision + SPC |
| Documentation | AS9102 report | Full DHR per 21 CFR 820 |
This disciplined workflow—anchored in predictive engineering, validated tooling, and closed-loop production—eliminates scrap rates below 50 PPM while accelerating time-to-market. Wuxi Lead’s infrastructure guarantees that every medical component shipped performs flawlessly in life-critical applications.
Why Choose Wuxi Lead Precision
Partner with Lead Precision for Unmatched Expertise in Medical Plastic Injection Molding
At Wuxi Lead Precision Machinery, we specialize in delivering precision-engineered solutions tailored to the stringent demands of the medical device industry. Our expertise in custom metal manufacturing directly supports high-performance plastic injection molding systems that meet the exacting standards of biocompatibility, repeatability, and regulatory compliance. When you partner with Lead Precision, you are not just sourcing components—you are aligning with a trusted manufacturer that understands the critical nature of medical applications.
Our engineering team brings over 15 years of experience in designing and producing mold bases, cavities, cores, and precision tooling from high-grade stainless steels and hardened alloys. Every product is manufactured under ISO 13485 and ISO 9001 certified processes, ensuring full traceability, cleanroom-compatible finishing, and micron-level accuracy. We utilize advanced CNC machining centers, wire EDM, and surface grinding technologies to achieve tight tolerances as tight as ±0.002 mm—essential for medical components such as connectors, housings, fluid handling systems, and surgical instrument parts.
What sets us apart is our end-to-end collaboration model. From initial design review to DFM analysis, material selection, and final inspection, we work closely with your engineering team to optimize manufacturability, reduce cycle times, and eliminate defects. Our facility in Wuxi, China, is equipped with state-of-the-art metrology equipment including CMMs, optical comparators, and roughness testers to validate every component before shipment. We also offer mold flow analysis support and rapid prototyping services to accelerate your time to market.
We serve global medical OEMs and contract manufacturers who demand consistency, scalability, and full documentation for FDA or CE submissions. Whether you require low-volume precision molds or high-cavitation systems for mass production, our team ensures durability, corrosion resistance, and seamless integration with your injection molding process.
Partnering with Lead Precision means gaining a manufacturing ally committed to quality, responsiveness, and technical excellence. We understand that in the medical field, failure is not an option—and neither is compromise.
| Specification | Detail |
|---|---|
| Material Options | 316L Stainless Steel, 17-4PH, 420SS, Tool Steels (e.g., H13, S136) |
| Tolerance | ±0.002 mm |
| Surface Finish | Ra ≤ 0.2 µm (polished to mirror finish if required) |
| Maximum Part Size | 800 x 600 x 500 mm |
| Certifications | ISO 9001, ISO 13485, RoHS compliant |
| Secondary Processes | Passivation, CIP cleaning, laser marking, coating |
| Lead Time | 15–25 days (depending on complexity) |
Contact us today to discuss your next medical injection molding project. Email us at [email protected] and receive a detailed technical consultation with our application engineers. Let Wuxi Lead Precision Machinery be your strategic partner in precision.
⚙️ Precision Cost Estimator
Estimate relative manufacturing effort based on tolerance.

