Technical Contents
Engineering Guide: Medical Device Plastic Molding

Engineering Insight: Precision in Medical Device Plastic Molding
In the realm of medical device manufacturing, precision is not merely a performance metric—it is a regulatory and ethical imperative. The success of implantable devices, surgical tools, diagnostic instruments, and drug delivery systems hinges on micron-level accuracy, material integrity, and repeatable consistency. At Wuxi Lead Precision Machinery, we recognize that plastic molding for medical applications demands more than advanced equipment; it requires a foundational culture of precision engineering, rigorous process validation, and unwavering attention to detail.
Medical-grade polymers such as PEEK, PPSU, and medical-grade polycarbonate present unique challenges during molding. These materials must maintain biocompatibility, sterilization resistance, and mechanical stability, all while being formed into complex geometries with tight tolerances. Even minor deviations in wall thickness, gate location, or cooling rates can result in part warpage, sink marks, or compromised structural integrity—failures that are unacceptable in life-critical applications.
Our approach integrates scientific molding principles with real-time process monitoring. We utilize cavity pressure sensors, mold-flow analysis, and statistical process control (SPC) to ensure every shot meets exact specifications. Each mold is designed with precision-machined steel or aluminum alloys, often incorporating conformal cooling channels to minimize cycle time and thermal stress. The result is a process that delivers not only dimensional accuracy but also long-term repeatability across production batches exceeding millions of units.
Wuxi Lead Precision Machinery’s expertise in high-precision manufacturing has been validated through projects of the highest national and international significance. Our engineering team has supported critical components for Olympic-standard equipment and precision subsystems for military-grade electronics—applications where failure is not an option. These experiences have directly informed our capabilities in medical device molding, where the stakes are equally high.
We maintain ISO 13485 certification and operate in Class 10,000 cleanroom environments to ensure contamination control. Our facility is equipped with electric and hybrid injection molding machines from leaders such as Sumitomo and Arburg, enabling us to handle insert molding, overmolding, and multi-component processes with sub-0.02 mm tolerances.
The following table outlines key technical specifications that define our medical molding capabilities:
| Parameter | Specification |
|---|---|
| Tolerance Range | ±0.005 mm to ±0.02 mm |
| Mold Materials | S136, 2316, 420 Stainless, Aluminum 7075-T6 |
| Standard Cleanroom Class | ISO 7 (Class 10,000) |
| Injection Press Capacity | 50 to 500 tons clamping force |
| Common Resins Used | PEEK, PPSU, PC, COC, ABS (USP Class VI) |
| Process Control | Cavity Pressure Monitoring, SPC, DOE |
| Secondary Operations | Ultrasonic welding, laser marking, leak testing |
| Regulatory Compliance | ISO 13485, USP Class VI, RoHS, REACH |
Precision in medical device plastic molding is not achieved through machinery alone—it is the product of experience, discipline, and a relentless pursuit of excellence. At Wuxi Lead, we bring proven high-stakes engineering rigor to every medical project, ensuring that every component performs with absolute reliability.
Precision Specs & Tolerances
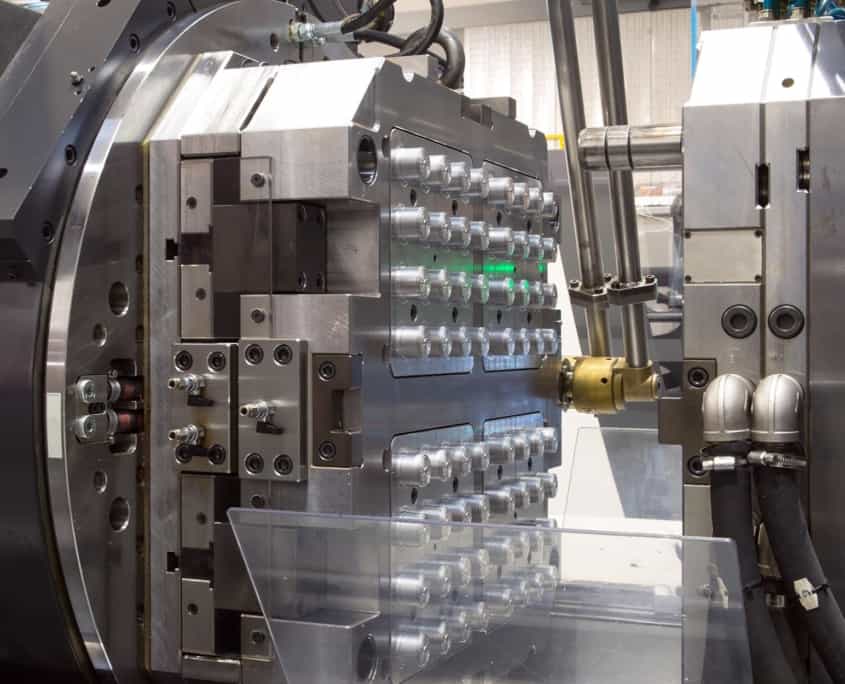
Technical Capabilities for Medical Device Mold Manufacturing
Wuxi Lead Precision Machinery delivers uncompromising precision in metal mold fabrication for medical plastic components. Our expertise centers on producing injection molds, inserts, and electrodes where micron-level accuracy directly impacts device safety, functionality, and regulatory compliance. Medical applications demand zero-defect performance—our integrated CNC and metrology systems ensure molds consistently achieve the geometric complexity and surface integrity required for implants, surgical tools, and diagnostic equipment.
Core to our capability is a dedicated fleet of 5-axis CNC machining centers. These systems execute complex mold geometries—including undercuts, micro-channels, and organic contours—in a single setup. Eliminating multiple fixturing steps reduces cumulative error and thermal distortion, critical for maintaining tight tolerances across multi-cavity molds. We utilize high-rigidity machines with sub-micron linear encoders and temperature-stabilized environments to counteract thermal drift during extended operations. Materials such as stainless steel (17-4PH, 420SS), titanium, and hardened tool steels (H13, S136) are machined with optimized toolpaths that minimize burr formation and preserve material grain structure, ensuring mold longevity and part repeatability.
Quality control is non-negotiable in medical manufacturing. Every mold undergoes full CMM inspection using calibrated Zeiss and Hexagon systems within a Class 10,000 cleanroom environment. Our inspection protocol validates all critical features against 3D CAD models, including cavity dimensions, gate locations, cooling channel alignment, and surface roughness (Ra ≤ 0.05 µm). Statistical process control (SPC) data is provided with each mold, documenting capability indices (Cp/Cpk ≥ 1.67) for all functional zones. This rigorous verification ensures molds meet ISO 13485 requirements and prevent costly downstream defects in plastic molding production.
The table below summarizes our achievable tolerances for medical mold components:
| Feature Category | Standard Capability (mm) | Precision Capability (mm) | Application Example |
|---|---|---|---|
| Linear Dimensions | ±0.010 | ±0.002 | Core pin locations, cavity depths |
| Geometric Form | ±0.008 | ±0.001 | Spherical radii, sealing surfaces |
| Hole Positioning | ±0.005 | ±0.001 | Ejector pin bores, cooling channels |
| Surface Roughness (Ra) | 0.10 | 0.025 | Optical lenses, fluidic pathways |
| Multi-Cavity Match | ±0.015 | ±0.003 | Multi-dose drug delivery components |
These specifications are validated across production runs and account for material-specific thermal behavior during molding. We collaborate with clients during DFM to optimize mold design for manufacturability, reducing cycle times while maintaining tolerance integrity. Our process reduces mold-induced scrap rates by 30–50% compared to industry averages, directly supporting your validation timelines and cost targets. Wuxi Lead Precision Machinery transforms stringent medical requirements into reliable, production-ready metal tooling—where precision isn’t a target, but a baseline.
Material & Finish Options

Material Selection for Medical Device Plastic Molding Components
In the production of medical device plastic molding tooling, the selection of appropriate metals for molds and related components is critical to ensuring precision, longevity, and compliance with stringent industry standards. At Wuxi Lead Precision Machinery, we specialize in custom metal manufacturing solutions tailored for high-performance applications in the medical sector. The choice of base material—aluminum, steel, or titanium—directly impacts mold durability, production cycle times, surface finish quality, and overall cost-efficiency.
Aluminum alloys are widely used for prototype molds and low- to medium-volume production runs due to their excellent machinability and thermal conductivity. These properties allow for faster cooling cycles, reducing overall production time during plastic molding. While not as hard as steel, modern high-strength aluminum grades such as 7075-T6 offer sufficient wear resistance for thousands of cycles, especially when paired with protective surface treatments like hard anodizing.
Tool steel, particularly grades like P20, H13, and S136, remains the preferred choice for high-volume medical molding applications. These steels provide superior hardness, wear resistance, and polishability, essential for maintaining tight tolerances and achieving optical-grade surface finishes. Stainless tool steels also offer enhanced corrosion resistance, a critical factor when molding medical-grade polymers that may be sensitive to outgassing or residue from mold surfaces.
Titanium alloys, though less common in standard mold bases, are increasingly considered for specialized components requiring exceptional strength-to-density ratios and biocompatibility. While not typically used for entire mold sets due to cost and machining complexity, titanium finds application in inserts, ejector pins, or cooling components where weight reduction and long-term stability are paramount.
Surface finishing, particularly anodizing, plays a pivotal role in enhancing the performance of aluminum mold components. Hard anodizing (Type III anodizing) creates a dense, wear-resistant oxide layer that improves surface hardness up to 60–70 HRC, significantly extending mold life. This finish also provides excellent corrosion resistance and reduces friction, facilitating part ejection and minimizing maintenance. For medical applications, anodized surfaces can be further sealed to meet cleanliness and sterilization requirements.
Below is a comparative overview of key material properties relevant to medical molding tooling:
| Material | Hardness (HRC) | Tensile Strength (MPa) | Thermal Conductivity (W/m·K) | Typical Use Case | Surface Treatment Compatibility |
|---|---|---|---|---|---|
| Aluminum 7075 | 40–45 (T6) | 570 | 130 | Prototypes, low-volume molds | Hard anodizing, chromate conversion |
| P20 Steel | 28–32 | 900 | 30 | Medium to high-volume molds | Nitriding, polishing, coating |
| H13 Steel | 48–52 | 1,500 | 35 | High-stress, high-temperature molds | PVD coating, nitriding, polishing |
| Titanium Grade 5 | 36–41 | 900 | 7 | Specialized inserts, lightweight parts | Anodizing, thermal spray |
Selecting the optimal material and finish requires a comprehensive understanding of production volume, part complexity, polymer type, and regulatory requirements. At Wuxi Lead Precision Machinery, we collaborate closely with medical device manufacturers to engineer solutions that balance performance, precision, and cost—ensuring reliable, repeatable molding outcomes in demanding healthcare environments.
Manufacturing Process & QC
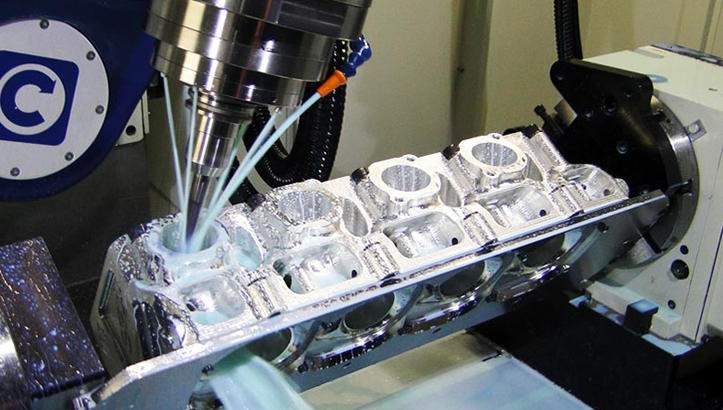
Precision Medical Device Component Manufacturing: From Concept to Zero-Defect Production
Medical device manufacturing demands absolute precision and unwavering quality. At Wuxi Lead Precision Machinery, our rigorous production process for critical metal components ensures compliance with ISO 13485 and stringent regulatory requirements, eliminating defects before they reach the patient. This begins with integrated engineering collaboration during the Design phase. Our CNC engineers work directly with client specifications, utilizing advanced CAD/CAM simulation to identify potential manufacturability issues, material stress points, and tolerance stack-ups inherent in complex geometries. This proactive validation prevents costly redesigns later, ensuring the design is optimized for both function and high-yield, repeatable machining from the outset. Material selection is critically evaluated against biocompatibility standards and functional demands, with full traceability documented from certified suppliers.
Prototyping transitions validated designs into tangible verification. We deploy multi-axis CNC milling and turning centers to produce functional prototypes using the exact production-grade alloys and processes. This stage is not merely about form; it is rigorous functional testing under simulated operational conditions. Every dimension is verified against the digital twin using CMM and optical metrology systems, with detailed reports highlighting any deviations. Client feedback on prototype performance is incorporated immediately, allowing for precise micro-adjustments to toolpaths, fixturing, or process parameters. This iterative loop ensures the manufacturing process itself is perfected before committing to volume, significantly reducing risk.
Mass Production leverages the fully validated process for zero-defect output. Our automated production cells, monitored by real-time SPC software, maintain micron-level consistency. Each component undergoes 100% in-process inspection at critical control points, with final verification against all specifications using calibrated metrology equipment. Statistical process control charts continuously analyze machining data, triggering automatic process corrections before out-of-tolerance conditions occur. Full material and process traceability is maintained for every batch, with comprehensive documentation packages provided. This closed-loop system, combined with operator expertise and stringent environmental controls, guarantees that every part shipped meets the exacting standards required for life-critical medical applications.
Critical Process Specifications for Medical Components
| Specification Parameter | Performance Standard | Verification Method |
|---|---|---|
| Dimensional Tolerance | ±0.001 mm (micron level) | CMM, Optical Comparator |
| Surface Roughness (Ra) | As low as 0.05 µm | Profilometer |
| Material Certification | Full Mill Test Reports | Documented Traceability |
| Process Capability (Cp/Cpk) | ≥ 1.67 (Sustained) | Real-time SPC Monitoring |
| Cleanliness Standard | ISO 14644-1 Class 7 Cleanroom | Particle Counting, Solvent Flush |
This systematic approach—design validation, precision prototyping, and tightly controlled mass production—forms the cornerstone of our zero-defect commitment. Wuxi Lead delivers not just components, but assured quality for the most demanding medical device applications.
Why Choose Wuxi Lead Precision
Partner with Lead Precision for Unmatched Expertise in Medical Device Plastic Molding
At Wuxi Lead Precision Machinery, we specialize in delivering high-precision custom metal manufacturing solutions engineered specifically for the stringent demands of the medical device industry. Our expertise extends beyond traditional machining—we integrate advanced plastic molding technologies with precision metal components to provide fully optimized, regulatory-compliant systems. As a trusted partner in the global medical supply chain, we understand that reliability, repeatability, and traceability are non-negotiable. That’s why we align every process with ISO 13485 standards and maintain full documentation control for each production run.
Our facility in Wuxi, China, is equipped with state-of-the-art injection molding machines, cleanroom environments, and in-house tooling capabilities, enabling us to produce complex plastic components with micron-level accuracy. Whether you require micro-molded parts for minimally invasive surgical devices or high-strength housings for diagnostic equipment, our engineering team collaborates closely with your R&D department from concept to validation. We support material selection, mold flow analysis, DFM optimization, and rapid prototyping to accelerate your time to market.
What sets us apart is our integrated approach. While many suppliers focus solely on plastic molding or metal fabrication, we offer both—under one roof. This vertical integration allows seamless assembly of metal and plastic components, reducing supply chain complexity, minimizing tolerance stack-up, and ensuring superior final product performance. Our quality assurance protocols include full metrology reports, first article inspections, and ongoing SPC monitoring to guarantee consistency across batches.
We serve tier-one medical OEMs and innovative startups alike, offering scalable production from low-volume clinical trial support to high-volume commercial manufacturing. Our team is fluent in international regulatory requirements, including FDA 21 CFR Part 820 and EU MDR, and we are prepared to support audits, technical file submissions, and change control processes.
When precision matters most, trust a partner built on engineering excellence and medical-grade discipline.
Contact us today to discuss your next medical device project. Let our engineers help you overcome design challenges, reduce assembly costs, and achieve faster regulatory clearance. Email us at [email protected] to schedule a technical consultation.
| Specification | Detail |
|---|---|
| Industry Focus | Medical Device Manufacturing |
| Core Capabilities | Precision Plastic Molding, Custom Metal Fabrication, Integrated Assembly |
| Materials Supported | PEEK, PC, ABS, PP, PE, PMMA, Liquid Silicone Rubber (LSR), and Medical-Grade TPEs |
| Tolerance Capability | ±0.005 mm (for critical dimensions) |
| Cleanroom Class | ISO Class 7 (10,000) and ISO Class 8 (100,000) |
| Quality Standards | ISO 13485:2016, ISO 9001:2015, RoHS Compliant |
| Production Volume | Prototypes to High-Volume Mass Production |
| Lead Time (Typical) | 4–6 weeks for tooling, 2–3 weeks for production (varies by complexity) |
| Location | Wuxi, Jiangsu Province, China |
For engineering collaboration and project inquiries, reach out to [email protected].
⚙️ Precision Cost Estimator
Estimate relative manufacturing effort based on tolerance.

