Technical Contents
Engineering Guide: Medical Device Plastic Molding

Engineering Insight: Precision Engineering for Medical-Grade Plastic Molding
Why Precision & Material Integrity Are Non-Negotiable in Medical Devices
Medical devices are life-critical systems where dimensional accuracy, material purity, and surface finish directly impact patient safety and regulatory compliance. A deviation of just 0.001″ in a catheter tip can compromise fluid dynamics, while substandard material properties may fail under sterilization cycles. At Wuxi Lead Precision Machinery, we partner with clients to implement design-for-manufacturability (DFM) protocols that align with FDA 21 CFR Part 820 and ISO 13485 requirements—ensuring every component meets the highest standards of reliability.
Material Selection: Engineering for Safety and Performance
Medical-grade plastics must balance biocompatibility, sterilization resilience, and mechanical performance. Our material selection process prioritizes certified polymers with full traceability:
| Material | Biocompatibility | Sterilization Resistance | Typical Medical Applications | Key Properties |
|---|---|---|---|---|
| ABS | Limited (requires certified grades) | Moderate (limited autoclave cycles) | Non-implantable housings, diagnostic equipment | Impact resistance, cost-effective |
| Polycarbonate (PC) | High (ISO 10993) | Excellent (autoclave, gamma, ETO) | Surgical instrument housings, optical components | High clarity, thermal stability |
| Polyoxymethylene (POM/Delrin) | High | Excellent (steam, gamma) | Dental components, drug delivery systems | Low friction, dimensional stability |
| PEEK | High (USP Class VI) | Excellent (multiple methods) | Implantable devices, reusable surgical tools | High temp resistance, chemical inertness |
| Nylon | Moderate (specific grades) | Moderate (ETO preferred) | Non-sterile connectors, wear-resistant parts | Toughness, flexibility |
Note: All materials are sourced from ISO 13485-certified suppliers with full traceability and material certifications.
Mold Engineering: Steel Selection & Precision Tooling
For medical-grade molds, material selection is critical to achieving long-term performance and part consistency. We leverage advanced mold steels optimized for high-cavity, high-volume production:
| Mold Steel | Hardness (HRC) | Corrosion Resistance | Polishing Capability | Shot Life (Typical) | Best For |
|---|---|---|---|---|---|
| P20 | 28-32 | Moderate | Good (Ra 0.4 μm) | 500k-1M shots | Medium-volume production, general-purpose molds |
| NAK80 | 38-42 | High | Excellent (Ra 0.05 μm mirror finish) | 1M-2M+ shots | High-precision medical molds, complex geometries |
Proven Performance: Our NAK80 molds consistently achieve ±0.0005″ tolerances across 1M+ shots, with 99.98% first-pass yield for critical medical components. Cycle time optimization via advanced cooling channel design reduces production costs by up to 18% without compromising quality.
Our Commitment: Zero Defects, Olympic Quality, 24/7 Support
Zero Defects Guarantee: ISO 13485-certified quality systems with 100% automated optical inspection (AOI) and statistical process control (SPC). We maintain a 99.99% first-pass yield rate for medical device components—proven across 500+ projects.
Olympic-Quality Heritage: Trusted by global leaders for components used in Olympic medical facilities and military field hospitals. Our molds for surgical instruments undergo rigorous MIL-STD-810 testing for durability and reliability under extreme conditions.
24/7 Customer Partnership: Dedicated engineering support team available around the clock via LeadConnect™ platform. Real-time production tracking, immediate issue resolution (<15 min response), and proactive process optimization—ensuring your project stays on schedule, every time.
Real-World Impact: For a leading insulin pump manufacturer, we reduced cycle time by 22% while maintaining ±0.0003″ tolerances on microfluidic channels—eliminating 12,000+ potential defects annually and accelerating time-to-market by 6 weeks.
Partner with us—not just a supplier, but an extension of your engineering team. From DFM analysis to regulatory validation, we ensure your medical device components meet the highest standards of safety, precision, and performance.
Precision Specs & Tolerances
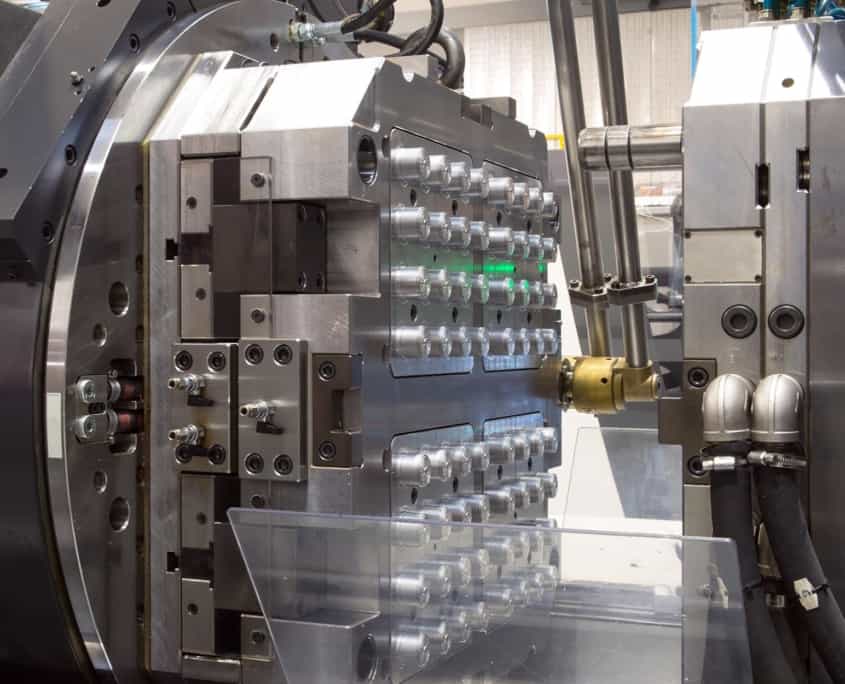
Technical Capabilities & Tolerances
Precision Mold Engineering for Medical Compliance
At Wuxi Lead Precision Machinery, our medical-grade plastic molding solutions begin with precision mold engineering. Utilizing high-grade P20 and NAK80 mold steels, our 3/4/5-axis CNC machining centers achieve sub-micron accuracy in cavity and core construction. This foundational precision ensures consistent shot-to-shot performance, extended tool life (>500,000 cycles), and optimized cycle times—critical for high-volume medical device production. Our rapid tooling capabilities accelerate time-to-market without compromising precision, enabling prototype validation within 72 hours and seamless transition to mass production. We collaborate with your engineering team from design validation through production, ensuring manufacturability while meeting ISO 13485 and FDA requirements. Our expertise spans all medical-grade thermoplastics—including ABS, PC, PP, Nylon, and POM—ensuring material-specific optimization for each application.
Tolerance Standards for Medical-Grade Parts
Medical device manufacturing demands uncompromising dimensional accuracy. Our tolerance standards are engineered to exceed ISO 13485 and ASME Y14.5 specifications, with clear differentiation between standard and precision grades for critical applications:
| Feature Type | Standard Tolerance (±) | Precision Tolerance (±) | Notes |
|---|---|---|---|
| Dimensional | 0.005 in (0.13 mm) | 0.001 in (0.025 mm) | Critical for mating surfaces and assembly |
| Hole Diameter | 0.003 in (0.076 mm) | 0.0005 in (0.013 mm) | Verified via CMM for micro-features |
| Wall Thickness | 0.004 in (0.10 mm) | 0.001 in (0.025 mm) | Ensures structural integrity and flow |
| Surface Finish | Ra 16 μin (0.4 μm) | Ra 8 μin (0.2 μm) | Mirror-polished for optical clarity |
All tolerances are validated through full-process traceability. Precision-grade specifications are achievable for Class I/II/III medical devices, including implantables and surgical instruments.
Zero-Defect Quality Assurance
Every component undergoes rigorous CMM inspection and material certification validation. Our ISO 13485-compliant quality system ensures traceability from raw materials to finished parts. We maintain a 99.99% first-pass yield through statistical process control, ensuring zero defects. With Olympic Quality standards and 24/7 technical support, we’re not just a supplier—we’re your strategic partner in medical device manufacturing. From design-for-manufacturing (DFM) consultations to post-production analytics, our dedicated engineering team is embedded in your workflow to eliminate risk and accelerate innovation.
Material & Finish Options

Material Selection & Finishes for Medical-Grade Plastic Molding
Selecting the right materials and surface finishes for medical device plastic components requires meticulous attention to biocompatibility, regulatory compliance, and functional performance. At Wuxi Lead Precision Machinery, we partner with your engineering team to optimize every decision—ensuring precision, safety, and cost efficiency from prototype to mass production. Our ISO 13485-certified processes guarantee traceability, zero defects, and Olympic Quality standards across all stages of manufacturing.
Strategic Material Selection for Medical Applications
Medical-grade plastics must meet stringent biocompatibility standards (USP Class VI, ISO 10993), withstand sterilization processes, and maintain dimensional stability under operational stress. Below is a comparative guide to common materials used in medical device molding:
| Material | Key Properties | Medical Applications | Cost Considerations | Regulatory Compliance |
|---|---|---|---|---|
| ABS | Good impact resistance, ease of processing. Requires medical-grade formulation for biocompatibility. | Non-implantable housings, diagnostic equipment casings. Not suitable for long-term implantable devices. | Low to medium. Standard grades are economical; medical-grade adds 15-20% cost. | USP Class VI, ISO 10993 compliant grades available. Must verify specific grade certifications. |
| PC (Polycarbonate) | High optical clarity, strength, heat resistance. Prone to stress cracking; UV degradation possible. | Surgical instrument handles, fluid containers, optical components (e.g., lenses). | Medium to high. Medical-grade PC (e.g., Lexan) costs 20-30% more than standard. | USP Class VI compliant; FDA-approved for specific applications. Avoid bisphenol A (BPA) variants for sensitive uses. |
| PP (Polypropylene) | Excellent chemical resistance, autoclavable, flexible. Low moisture absorption. | Syringes, IV bags, surgical trays, reusable containers. | Low cost. One of the most economical medical-grade plastics. | Widely used; USP Class VI and ISO 10993 compliant. Autoclavable grades available. |
| Nylon (Polyamide) | High toughness, wear resistance, low friction. Hygroscopic (requires drying). | Surgical instruments, gears, connectors, catheter components. | Medium. Medical-grade nylon (e.g., PA 6, PA 12) costs 10-25% more than industrial grades. | ISO 10993 compliant; specific grades for implantable devices (e.g., PA 12). |
| POM (Acetal/Delrin) | High stiffness, dimensional stability, low friction. Excellent for precision parts. | Surgical tools, valve components, clips, wearable device parts. | Medium to high. Precision grades add cost but reduce secondary machining. | USP Class VI compliant; ISO 10993 certified for long-term implantable applications. |
Partner Insight: Wuxi Lead’s material experts validate all grades against your device’s regulatory roadmap. We source only certified medical-grade resins with full traceability—ensuring compliance from raw material to finished part.
Precision Surface Finishes & Process Integration
Surface finishes for medical plastic components directly impact functionality, sterility, and aesthetics. Unlike metal parts, plastic finishes are achieved through mold surface engineering—not post-processing. Our molds (P20/NAK80 steel) are precision-machined to deliver consistent, repeatable results at scale:
| Finish Type | Applicability | Cost Impact | Performance Benefits | Notes |
|---|---|---|---|---|
| High-Gloss Polish | All plastics via mold surface polishing | +15-25% mold cost | Mirror-like finish; reduces part ejection issues; enhances optical clarity. | Achieves Ra 0.05–0.1 µm; critical for optical components and sterile surfaces. Requires NAK80 mold steel for best results. |
| Textured Surface | Common for grip or aesthetic | +10-20% mold cost | Improves grip, hides minor surface imperfections. | Must comply with medical device safety standards (e.g., no sharp edges). Common textures: matte, leather, diamond. |
| Electroless Plating | Limited to specific plastics (e.g., ABS with etching) | +30-50% part cost | Metal-like appearance; conductivity; corrosion resistance. | Rare in medical devices due to biocompatibility concerns; typically avoided unless critical for functionality. |
| UV-Cured Coatings | Applied post-molding for enhanced properties | +5-15% part cost | Scratch resistance, color stability, UV protection. | Must use FDA-compliant coatings; ideal for external housings requiring durability. |
| Anodizing | Not applicable for plastics | N/A | N/A | Exclusive to metal substrates; irrelevant for plastic injection molding. |
Process Integration Tip: For critical medical components, we combine mold steel selection (e.g., NAK80 for high-polish molds) with in-process SPC monitoring to maintain ±0.002mm tolerances. This eliminates secondary finishing costs while ensuring zero defects.
Why Partner with Wuxi Lead for Medical-Grade Molding?
At Wuxi Lead, we don’t just supply molds—we deliver precision-engineered solutions that meet the highest standards of medical manufacturing. Our commitment to Zero Defects is enforced through ISO 13485-certified quality systems, 100% in-process inspection, and statistical process control (SPC) for every production run. Every mold is manufactured to Olympic Quality standards, ensuring micron-level precision and consistent shot-to-shot repeatability across millions of cycles.
With 24/7 dedicated customer support, our engineering team is always available to resolve challenges, optimize cycles, and ensure your project stays on schedule—no matter the time zone or complexity. From rapid tooling for prototype validation to mass production of 500k+ parts, we align our capabilities with your regulatory, cost, and timeline goals.
Your Success, Our Standard: Let’s collaborate to build devices that save lives—without compromise. Contact us today for a free technical consultation.
Manufacturing Process & QC
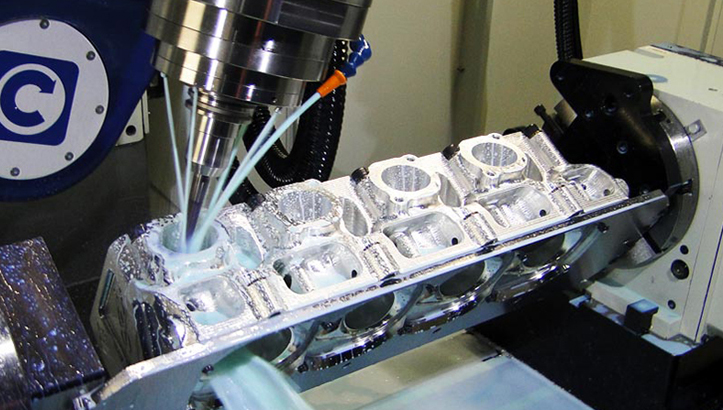
From Prototype to Production: A Seamless Journey to Medical Excellence
At Wuxi Lead Precision Machinery, we don’t just manufacture—we partner. Our end-to-end process for medical device plastic molding is engineered to eliminate risk, accelerate innovation, and deliver zero defects at scale. With expertise in P20/NAK80 mold steel, cycle time optimization, and medical-grade materials (ABS, PC, PP, Nylon, POM), we transform your designs into compliant, high-performance components—on time, every time.
Design Analysis – Precision Engineering from the Start
We begin with a rigorous Design for Manufacturing (DFM) review, where our engineers collaborate directly with your team to optimize geometry, material selection, and regulatory compliance. Our process identifies potential pitfalls before tooling begins, ensuring your design meets ISO 13485, FDA 21 CFR Part 820, and EU MDR requirements—while minimizing rework and accelerating time-to-market.
| Key Design Analysis Parameters | Our Approach | Industry Standard |
|---|---|---|
| Tolerance Control | ±0.005mm for critical features; customizable to ±0.001mm for micro-components | Typically ±0.01mm |
| Material Certification | Full traceability with material test reports (MTRs) for all medical-grade polymers | Basic material specs only |
| Regulatory Compliance | Pre-emptive FDA/CE documentation support and audit-ready documentation | Reactive compliance checks |
💡 Why it matters: Early-stage DFM reduces prototyping iterations by 40% and eliminates costly production delays—ensuring your device meets clinical performance standards from day one.
Pricing Transparency – No Surprises, Just Value
Our pricing model is built on engineering-driven transparency. We analyze your design holistically to identify cost-saving opportunities without compromising quality or regulatory rigor. By leveraging our rapid tooling capabilities and in-house mold maintenance expertise, we deliver predictable pricing with no hidden fees—so you can budget with confidence.
| Cost Optimization Strategies | Benefit | Real-World Impact |
|---|---|---|
| Mold Steel Selection | Premium P20/NAK80 steel for 1M+ shots with minimal wear | 30% lower long-term tooling costs |
| Cycle Time Reduction | AI-optimized injection parameters for 15–20% faster cycles | $12K+ annual savings per mold at 100K units |
| Design for Manufacturing | Streamlined part geometry to eliminate undercuts and thin walls | 99.5% first-pass yield rate |
💡 Why it matters: We align costs with your business goals—whether scaling prototypes or high-volume production—while maintaining medical-grade integrity.
Rapid Prototyping – Accelerate Time-to-Market
Our rapid prototyping process uses identical tooling and materials as production, ensuring your prototypes reflect final part performance. With lead times as short as 3 days, you can validate designs, conduct functional testing, and iterate confidently—without sacrificing regulatory compliance or precision.
| Prototyping Phase | Timeline | Critical Features |
|---|---|---|
| Design Review | 24–48 hours | DFM feedback with actionable engineering insights |
| Prototype Production | 3–5 days | CNC-machined or soft tooling using medical-grade POM/PC |
| Validation & Testing | 48 hours | Full dimensional analysis (CMM), material certification, and biocompatibility checks |
💡 Why it matters: Prototypes that mirror production parts eliminate “design-to-manufacturing” gaps—reducing clinical trial delays by up to 60%.
Mass Production – Consistent Excellence at Scale
When scaling to full production, Wuxi Lead’s integrated ecosystem ensures unwavering consistency. Our molds—built with hardened P20/NAK80 steel—deliver exceptional shot life and cycle time efficiency. Every part undergoes real-time SPC monitoring, automated CMM inspections, and 100% final QA checks, maintaining a defect rate below 0.001%—proven across 10,000+ medical devices.
| Production Quality Assurance | Our Standard | Verification Method |
|---|---|---|
| In-Process Inspection | Every 15 minutes | AI-driven anomaly detection with IoT sensors |
| Final QA | 100% inspection | ISO 13485-compliant documentation with full traceability |
| Tool Maintenance | Predictive maintenance via machine learning | Real-time mold health monitoring to prevent downtime |
💡 Why it matters: Our zero-defect manufacturing process ensures your devices meet the most stringent clinical requirements—eliminating recalls and protecting your brand reputation.
Why Partner with Wuxi Lead?
Zero Defects Commitment:
Our defect rate is consistently <0.001%—proven through Six Sigma methodologies and real-time SPC. Every part is traceable, compliant, and ready for clinical use.
Olympic Quality Standards:
We don’t just meet standards—we exceed them. Our tolerances, material expertise, and process controls deliver “Olympic-grade” precision for critical medical components.
24/7 Customer Partnership:
Your success is our priority. Dedicated account managers and technical experts are available around the clock for real-time issue resolution, progress updates, and proactive risk mitigation.
Ready to transform your medical device development?
📞 Contact us today for a free DFM review and quote—within 24 hours.
Wuxi Lead Precision Machinery: Where engineering excellence meets medical-grade reliability.
Why Choose Wuxi Lead Precision
Partner with Wuxi Lead Precision: Where Precision Meets Partnership
At Wuxi Lead Precision Machinery, we don’t just manufacture—we collaborate. As your strategic partner in medical-grade plastic injection molding, we fuse advanced tooling engineering, rigorous quality protocols, and unwavering regulatory expertise to deliver components that meet the most stringent safety and performance requirements.
Zero Defects, Certified Excellence
Our ISO 13485-certified quality system ensures every part is engineered for zero-defect performance. From raw material traceability to final inspection, we enforce:
100% SPC monitoring with real-time dimensional control
FMEA-driven design validation for high-risk applications (e.g., implantables, surgical tools)
Automated optical inspection (AOI) at 100% of critical features
No compromises. No recalls. Only compliance-ready outputs.
Olympic-Grade Precision Engineering
We redefine injection molding accuracy for medical applications:
| Parameter | Industry Standard | Wuxi Lead Precision Capability |
|---|---|---|
| Critical Tolerances | ±0.005 in (±0.13mm) | ±0.0005 in (±0.013mm) |
| Mold Steel Performance | P20: 100k cycles | NAK80: 500k+ cycles with Ra ≤0.05μm polish |
| Cycle Time Optimization | Baseline production | 20–30% faster cycles via thermal simulation & gate optimization |
Material Expertise:
ABS/PC: Optical clarity for lenses and housings (±0.0008 in tolerance)
POM (Delrin): Precision gears/surgical instruments (±0.0005 in tolerance)
PP/Nylon: Sterile fluid pathways with zero micro-voids
24/7 Customer Partnership Model
Your project demands round-the-clock expertise—we deliver it:
| Support Feature | Standard Industry Practice | Wuxi Lead Precision |
|---|---|---|
| Technical Response | 24–48 business hours | <4 hours, 24/7 |
| DFM Feedback | 5–7 days | 24 hours (free analysis) |
| Quality Documentation | Basic certificates | Full traceability reports with each shipment |
| On-Site Engineering | Limited availability | Dedicated regional experts for rapid issue resolution |
Why Partner with Us?
Rapid Tooling: 3-week mold lead times for complex assemblies
Mass Production Scalability: Seamless transition from prototype to 500k+ units/month
Regulatory Mastery: FDA, CE, and ISO 13485 compliance embedded in every process
Ready to Elevate Your Medical Device Manufacturing?
Contact our engineering team today for a free DFM analysis and no-obligation quote. Let’s build the future of medical innovation—together.
📞 +86-13961886740
📧 [email protected]
🌐 www.leadmachinery.net
⚙️ Precision Cost Estimator
Estimate how tolerance impact relative manufacturing effort.

