Technical Contents
Engineering Guide: Medical Injection Moulding

Engineering Insight: Precision in Medical Injection Moulding
In the field of medical device manufacturing, precision is not merely a performance metric—it is a regulatory and functional imperative. Medical injection moulding demands micron-level accuracy, repeatable process control, and uncompromising material integrity. Components such as syringe bodies, catheter hubs, and surgical instrument housings require tight tolerances, often within ±0.005 mm, to ensure compatibility, sterility, and patient safety. At Wuxi Lead Precision Machinery, we understand that in medical applications, even the smallest dimensional deviation can lead to functional failure or regulatory non-compliance.
Our engineering approach integrates advanced CNC machining with real-time process monitoring to produce moulds that deliver consistent, high-fidelity parts across large production runs. We utilize hardened tool steels and corrosion-resistant alloys, precision-ground and polished to mirror finishes (Ra < 0.025 µm), to support clean demoulding and reduce microbial adhesion—critical for implantable and diagnostic devices. Each mould undergoes rigorous metrology validation using coordinate measuring machines (CMM) and optical profilometry, ensuring geometric accuracy before deployment.
What sets Wuxi Lead apart is our proven track record in high-stakes precision manufacturing. Our engineering team has delivered mission-critical components for Olympic-grade timing systems and military-grade communication enclosures—applications where failure is not an option. These experiences have honed our ability to manage thermal stability, material flow dynamics, and multi-cavity synchronization in injection moulding, directly transferring into superior performance in medical-grade production.
We specialize in complex, multi-component moulds with micro-features, side actions, and hot runner systems designed for medical-grade resins such as PEEK, PSU, and implantable-grade polycarbonate. Our in-house design-to-production workflow enables rapid prototyping, Design for Manufacturability (DFM) analysis, and full documentation traceability—aligning with ISO 13485 and FDA requirements.
The table below outlines key performance specifications achievable through our medical injection moulding solutions:
| Parameter | Specification |
|---|---|
| Dimensional Tolerance | ±0.005 mm typical |
| Surface Finish (Cavity) | Ra < 0.025 µm (mirror polish) |
| Tool Steel Options | S136, 420SS, H13, Maraging Steel |
| Mould Life | 1,000,000+ cycles (optimized cooling design) |
| Cavity Configuration | 1–16 cavities, family or single-part molds |
| Hot Runner Systems | Fully validated, medical-grade nozzles |
| Cleanroom Moulding Available | ISO Class 7 compliant |
| Metrology Validation | 100% CMM and vision inspection reporting |
At Wuxi Lead Precision Machinery, we combine decades of custom metal manufacturing expertise with a deep understanding of medical industry demands. Our commitment to engineering excellence ensures that every mould we produce meets the highest standards of precision, durability, and regulatory compliance. For medical OEMs, this translates into reduced risk, accelerated time-to-market, and long-term production reliability.
Precision Specs & Tolerances
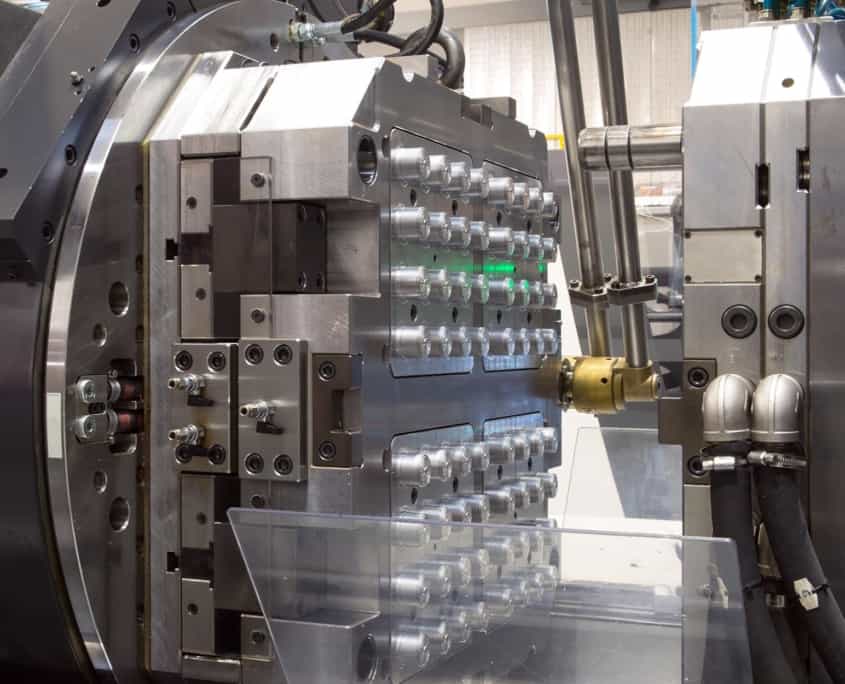
Technical Capabilities for Medical Injection Moulding Tooling
Wuxi Lead Precision Machinery delivers uncompromising precision for medical injection moulding tooling, where dimensional accuracy directly impacts patient safety and regulatory compliance. Our integrated manufacturing ecosystem centers on advanced 5-axis CNC machining capabilities, specifically engineered to produce complex mould cores, cavities, and inserts for demanding medical applications. These HAAS and DMG MORI systems achieve sub-micron repeatability while machining hardened tool steels (H13, S136, 420SS) and exotic alloys required for biocompatible part production. The simultaneous 5-axis motion eliminates secondary operations, ensuring critical undercuts, micro-features, and conformal cooling channels are machined in a single setup. This reduces cumulative error risks inherent in multi-stage processing—a non-negotiable requirement for Class I-III medical device components with features as small as 0.1mm wall thickness.
Rigorous quality control is embedded at every stage, anchored by Zeiss CONTURA G2 Coordinate Measuring Machines (CMM) with 0.5µm volumetric accuracy. Each tool undergoes full geometric dimensioning and tolerancing (GD&T) verification against ASME Y14.5 standards, with traceable reports meeting ISO 13485 and FDA 21 CFR Part 820 requirements. Our CMM protocols specifically validate critical attributes like gate geometry consistency, parting line flatness, and vent depth uniformity—factors directly influencing flash control and material flow in sterile medical moulding. Surface integrity is equally prioritized; we achieve mirror finishes (Ra ≤ 0.05µm) via optimized toolpaths and diamond polishing, minimizing bacterial adhesion points in implantable device moulds.
Material certification and thermal stability management form the foundation of our process. All tool steels undergo third-party chemical composition verification and vacuum heat treatment with controlled distortion rates below 0.02mm. In-process thermal monitoring during machining prevents microstructural deviations, while post-machining stress relief cycles ensure long-term dimensional stability under repeated sterilization cycles. This holistic approach guarantees moulds maintain tolerances through 500,000+ cycles—critical for high-volume diagnostic consumables or surgical instrument housings.
Critical Tolerance Specifications for Medical Mould Components
| Feature Category | Standard Capability | Medical-Grade Capability | Measurement Method |
|---|---|---|---|
| Linear Dimensions | ±0.010 mm | ±0.002 mm | CMM with 0.5µm probe |
| Geometric Form (Flatness, Roundness) | ±0.005 mm | ±0.001 mm | CMM + Optical Comparator |
| Micro-Features (Gates, Vents) | ±0.005 mm | ±0.001 mm | Confocal Microscopy |
| Surface Roughness (Ra) | 0.10 µm | 0.05 µm | Profilometer |
This precision framework enables reliable production of catheter hubs, drug delivery components, and surgical instrument moulds where micron-level deviations could compromise biocompatibility or functional performance. Wuxi Lead’s technical infrastructure ensures your medical tooling meets the exacting standards of global healthcare regulators while optimizing total production economics.
Material & Finish Options

Material Selection in Medical Injection Moulding: Precision for Performance
In the field of medical injection moulding, material selection is a critical determinant of tooling performance, longevity, and product compliance. At Wuxi Lead Precision Machinery, we specialize in custom metal manufacturing for high-precision moulds used in sterile, regulatory-intensive environments. The choice between aluminum, steel, and titanium must balance machinability, thermal conductivity, wear resistance, and biocompatibility. Each material offers distinct advantages depending on production volume, part complexity, and sterilization requirements.
Aluminum alloys, particularly 7075 and 6061-T6, are widely used for prototype and low-to-medium volume production moulds. Their high thermal conductivity enables faster cycle times, reducing cooling periods and accelerating time-to-market for medical devices. Aluminum is also easier to machine, allowing for intricate geometries and tight tolerances with reduced tool wear. However, its lower hardness compared to steel limits its use in high-volume runs where surface durability is paramount.
Tool steels such as H13, P20, and S136 are the standard for high-volume medical moulding applications. These steels offer superior hardness, wear resistance, and polishability—critical for achieving optical-grade surface finishes and maintaining dimensional stability over millions of cycles. Stainless variants like S136 also provide excellent corrosion resistance, essential for moulds exposed to aggressive cleaning agents or steam sterilization in cleanroom environments.
Titanium alloys, while less common due to higher cost and machining complexity, are gaining traction in specialized medical applications. Their exceptional strength-to-density ratio, biocompatibility, and resistance to corrosion make them ideal for implants or components requiring direct patient contact. When used in mould cores or inserts, titanium can enhance part integrity without introducing contamination risks.
Surface finishing further enhances material performance. Anodizing, particularly hard anodizing (Type III), is a key post-machining process for aluminum mould components. This electrochemical treatment builds a thick, wear-resistant oxide layer that improves surface hardness up to 60 HRC, increases corrosion resistance, and provides electrical insulation. For medical applications, anodized surfaces can be sealed in deionized water or Teflon-impregnated solutions to meet biocompatibility and non-stick requirements. While anodizing is specific to aluminum, steel components are often polished to mirror finishes (SPI A1) or coated with PVD/CVD layers for similar performance gains.
The following table summarizes key properties of commonly used materials in medical injection moulding:
| Material | Hardness (HRC) | Thermal Conductivity (W/m·K) | Corrosion Resistance | Typical Use Case |
|---|---|---|---|---|
| Aluminum 7075 | 15–20 | 130 | Moderate | Prototyping, low-volume production |
| H13 Steel | 48–52 | 35 | Good | High-volume moulds, hot runners |
| S136 Stainless | 50–54 | 25 | Excellent | Cleanroom, sterile environment tools |
| Titanium Grade 5 | 36–40 | 7 | Outstanding | Implant tooling, corrosive environments |
Material and finish selection must align with ISO 13485 standards and FDA guidelines for medical device manufacturing. At Wuxi Lead Precision Machinery, we support clients in matching material properties with application demands, ensuring every mould meets the highest benchmarks in precision, safety, and repeatability.
Manufacturing Process & QC
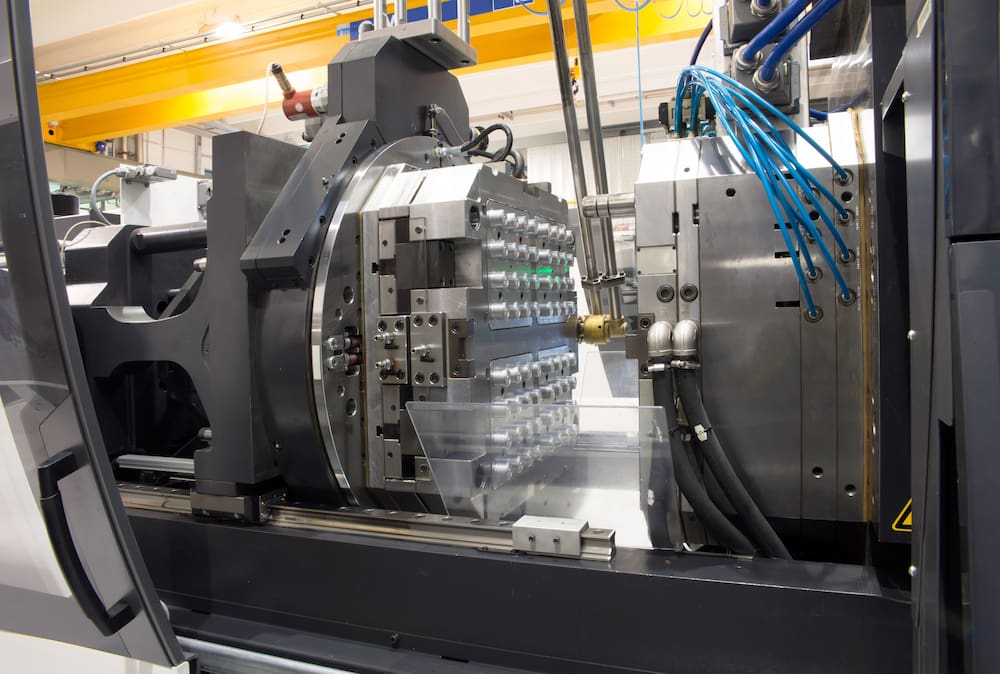
Medical Injection Moulding: Precision Production Process for Zero Defects
At Wuxi Lead Precision Machinery, our medical injection moulding process is engineered from inception to delivery for absolute defect elimination. Serving global medical device OEMs, we integrate stringent controls across Design, Prototyping, and Mass Production phases, ensuring components meet ISO 13485, USP Class VI, and biocompatibility standards. This systematic approach prevents failures before they occur, safeguarding patient safety and accelerating time-to-market.
Design Phase: Engineering Precision at the Foundation
Our process begins with collaborative digital engineering. Utilizing advanced CAD/CAM software and finite element analysis (FEA), we optimize part geometry, gate locations, and cooling channels to mitigate warpage, sink marks, and residual stress. Material selection is validated against biocompatibility requirements and sterilization methods, with full traceability from certified medical-grade resins. Mold flow simulation predicts fill patterns and pressure distribution, enabling proactive correction of potential knit lines or air traps. Critical tolerances down to ±0.005 mm are established and verified through virtual prototyping, ensuring manufacturability aligns with functional demands before metal is cut.
Prototyping Phase: Validating Performance Under Real Conditions
Rapid prototyping employs hardened steel molds machined via our 5-axis CNC centers, replicating production conditions rather than soft tooling. Each prototype undergoes rigorous dimensional inspection using CMMs and optical comparators against the validated digital model. Functional testing includes burst pressure, seal integrity, and assembly trials under simulated clinical use. Material lot traceability is maintained, and process parameters are locked using Design of Experiments (DOE) to identify optimal injection speed, pressure, and temperature windows. This phase confirms zero deviations from specifications and provides data for final process validation documentation required by regulatory bodies.
Mass Production Phase: Sustained Perfection Through Closed-Loop Control
Full-scale production operates within ISO Class 8 cleanrooms under strict environmental controls. Our molding cells feature real-time sensor monitoring of cavity pressure, melt temperature, and clamp force, feeding data into statistical process control (SPC) systems. Automated vision inspection checks 100% of critical features against tolerance limits, with immediate machine shutdown for out-of-spec conditions. Every batch includes destructive testing per AQL 0.065, with full material certificates and process validation reports. Continuous improvement is driven by Pareto analysis of any non-conformities, ensuring the process remains in a permanent state of zero-defect capability.
The following table details critical specifications maintained throughout our production continuum:
| Phase | Critical Parameters | Validation Methods | Defect Prevention Tactics |
|---|---|---|---|
| Design | ±0.005 mm tolerances, Mold flow balance | FEA, Mold Flow Simulation, Material Testing | Virtual defect elimination, DOE parameter lock |
| Prototyping | Dimensional accuracy, Functionality | CMM, Optical Inspection, Burst Testing | Hardened steel tooling, Real production parameters |
| Mass Production | Real-time cavity pressure, Cleanroom ISO 8 | SPC, 100% Automated Vision, AQL 0.065 | Closed-loop control, Immediate process abort |
This integrated methodology transforms design intent into flawless medical components. By embedding quality at every stage—not inspecting it in—we deliver consistent, regulatory-compliant production where zero defects is not a target, but the only acceptable outcome. Partner with Wuxi Lead to eliminate risk in your critical medical device supply chain.
Why Choose Wuxi Lead Precision
Partner with Lead Precision for Unmatched Excellence in Medical Injection Moulding
When precision, reliability, and regulatory compliance are non-negotiable, Wuxi Lead Precision Machinery stands as a trusted partner in custom metal manufacturing for the medical device industry. With over 15 years of engineering expertise, we specialize in delivering high-precision injection moulding solutions tailored to the stringent demands of medical applications. From concept to production, our end-to-end manufacturing capabilities ensure that every component meets exacting standards for biocompatibility, dimensional accuracy, and long-term performance.
Our advanced CNC machining centers, combined with cleanroom-compatible moulding technologies, enable us to produce complex, micro-scale components used in surgical instruments, diagnostic devices, implantables, and drug delivery systems. Every project is executed under ISO 13485-certified quality management protocols, ensuring traceability, material integrity, and full compliance with FDA and EU MDR requirements. At Lead Precision, we understand that medical devices demand more than precision—they require accountability, documentation, and repeatable consistency across production batches.
We collaborate closely with global OEMs, contract manufacturers, and R&D teams to engineer solutions that balance performance, cost-efficiency, and time-to-market. Our in-house tooling department allows rapid prototyping and DFM optimization, reducing development cycles and minimizing risk during scale-up. Whether you require 100 prototype units or high-volume production runs with automated inspection, our flexible manufacturing model adapts to your needs without compromising quality.
Our technical team brings deep experience in high-performance polymers such as PEEK, PPSU, PEI, and medical-grade silicones—materials that demand specialized processing knowledge. We integrate real-time process monitoring, statistical process control (SPC), and 100% vision inspection where required, ensuring defect prevention and maximum yield. All components are manufactured in controlled environments, with full material certification and batch documentation provided as standard.
Below are key technical capabilities that define our medical injection moulding services:
| Specification | Detail |
|---|---|
| Moulding Tolerances | Up to ±0.005 mm |
| Part Weight Range | 0.01 g – 300 g |
| Mould Base Materials | H13, S136, 420 Stainless Steel, Titanium Alloys |
| Standard Polishing | SPI A1, A2, B1 (cleanroom compatible) |
| Cleanroom Moulding | Class 10,000 (ISO 7) available |
| Injection Press Capacity | 50–1,200 tons |
| Secondary Operations | Ultrasonic welding, laser marking, assembly, packaging |
| Quality Standards | ISO 9001, ISO 13485, RoHS, USP Class VI compliant |
At Wuxi Lead Precision Machinery, we don’t just manufacture parts—we deliver engineering confidence. Our commitment to innovation, transparency, and technical partnership sets us apart in the global medical manufacturing landscape.
Contact us today at [email protected] to discuss your next medical injection moulding project. Let Lead Precision be the foundation of your product’s success.
⚙️ Precision Cost Estimator
Estimate relative manufacturing effort based on tolerance.

